Chemistry
Chemistry
PURIFICATION OF IMPURE COPPER BY ELECTROLYTIC PROCESS AND HALF-CELL EQUATIONS.
TOPIC: PURIFICATION OF IMPURE COPPER BY ELECTROLYTIC PROCESS AND HALF-CELL EQUATIONS.
OBJECTIVES:
- State the electrolyte used in the purification of impure copper.
- State the electrodes used in the process.
- Write down half cell equation for
- Cl−
- Mg2+
- OH−

Materials for the process:
Impure copper is the anode while pure copper is the cathode. The electrolyte is aqueous copper (II) tetraoxosulphate (VI). The current is allowed to follow for several hours.
As the process continues, the impure anode dissolves in the electrolyte thus;
Cu(s) – 2e− Cu2+(aq)
The copper ion migrates to the cathode and it is preferentially discharged
Cu2+(aq) + 2e− Cu(s)
The copper solid collects at the pure cathode while the impurity collects at the bottom of the cell as sludge. The impure copper then becomes pure.
Half-cell equation:
This equation represents the cathodic or anodic reaction in electrolytic process. E.g.
Reduction half-cell equation:
K+(aq) + e− K(s)
Ag+(aq) + e− Ag(s)
Ca2+(aq) + 2e− Ca(s)
Mg2+(aq) + 2e− Mg(s)
Oxidation half-cell equation:
4OH−(aq) – 4e− 2H2O(l) + O2(g)
2Cl−(aq) − 2e− Cl2(g)
ASSIGNMENT:
- State the electrolyte and electrodes used in the purification of impure copper using electrolytic process.
- Write half-cell equation for Cl−, OH−, and Mg2+
BASIC DEFINITIONS IN ELECTROLYTIC PROCESS
One of the most important properties of water is its ability to dissolve a wide variety of substances. Solutions in which water is the dissolving medium are called aqueous solutions. For electrolytes, water is the most important solvent. Ethanol, ammonia, and acetic acid are some of the non-aqueous solvents that are able to dissolve electrolytes.
Electrolytes
Substances that give ions when dissolved in water are called electrolytes. They can be divided into acids, bases, and salts, because they all give ions when dissolved in water. These solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution.
Electrolytes in Body Fluids
Our body fluids are solutions of electrolytes and many other things. The combination of blood and the circulatory system is the river of life, because it coordinates all the life functions. When the heart stops pumping in a heart attack, the life ends quickly. Getting the heart restarted as soon as one can is crucial in order to maintain life.
The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). These are nutritionally called macrominerals.
Electrolyte balance is crucial to many body functions. Here's some extreme examples of what can happen with an imbalance of electrolytes: elevated potassium levels may result in cardiac arrhythmias; decreased extracellular potassium produces paralysis; excessive extracellular sodium causes fluid retention; and decreased plasma calcium and magnesium can produce muscle spasms of the extremities.
When a patient is dehydrated, a carefully prepared (commercially available) electrolyte solution is required to maintain health and well being. In terms of child health, oral electrolyte is need when a child is dehydrated due to diarrhea. The use of oral electrolyte maintenance solutions, which is responsible for saving millions of lives worldwide over the last 25 years, is one of the most important medical advances in protecting the health of children in the century, explains Juilus G.K. Goepp, MD, assistant director of the Pediatric Emergency Department of the Children's Center at Johns Hopkins Hospital. If a parent provides an oral electrolyte maintenance solution at the very start of the illness, dehydration can be prevented. The functionality of electrolyte solutions is related to their properties, and interest in electrolyte solutions goes far beyond chemistry.
Electrolytes and Batteries
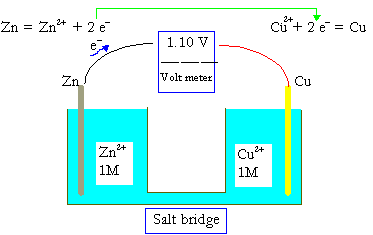 Solutions of electrolytes are always required in batteries, even in dry cells. The simplest battery consists of two electrodes. The figure here illustrates a copper-zinc battery. The left hand is a zinc electrode. The zinc atoms have a tendency to become ions, leaving the electrons behind.
Solutions of electrolytes are always required in batteries, even in dry cells. The simplest battery consists of two electrodes. The figure here illustrates a copper-zinc battery. The left hand is a zinc electrode. The zinc atoms have a tendency to become ions, leaving the electrons behind.
Zn(s)→Zn2+(aq)+2e−.
As the zinc ions going into the solution, anions move from the copper cell to the zinc cell to compensate for the charge, and at the same time, electrons go from the Zn electrode to the Cu electrode to neutralize the copper ions.
Cu2+(aq)+2e−→Cu(s)
In dry cells, the solution is replaced by a paste so that the solution will not leak out of the package. In this cell, the Zn and Cu electrode has a voltage of 1.10 V, if the concentrations of the ions are as indicated.
Chemical Reactions of Electrolytes
When solutions of electrolytes are combined, the cations and anions will meet each other. When the ions are indifferent of each other, there is no reaction. However, some cations and anions may form a molecule or solid, and thus the cations and anions change partners. These are called metathesis reactons, which include:
- Solid formation (or precipitation) reactions: the cations and anions form a less soluble solid, resulting in the appearance of a precipitate.
- Neutralization reactions: H+ of an acid and OH− of a base combine to give the neutral water molecule.
- Gas formation reactions: When neutral gaseous molecules are formed in a reaction, they leave the solution forming a gas.
Redox reactions are also possible between the various ions. In fact, the battery operations involve redox reactions.
ASSIGNMENT:
1. Define electrolyte
2. state the types of reactions that occur in cathode and anode.
3.Define
i. Strong electrolyte
ii. Weak electrolyte
Mr. Kaiwedo Stephen
Submit your assignment to the teacher via email:This email address is being protected from spambots. You need JavaScript enabled to view it.
For further inquiry call him on: 08038960651
Metallic Bond and Physical properties of Metals
Learning Objective
Metallic bond?
- Metallic bond is electrostatic force of attraction between mobile valence electrons of metals and positive charge.
Key Points
- Atoms in metals lose electrons to form cations. Delocalized electrons surround the ions. Metallic bonds (electrostatic interactions between the ions and the electron cloud) hold the metallic solid together. Atoms are arranged like closely packed spheres.
- Because outer electrons of metal atoms are delocalized and highly mobile, metals have electrical and thermal conductivity. The free electron model can be used to calculate electrical conductivity as well as the electrons’ contribution to the heat capacity and heat conductivity of metals.
- Metals are ductile, or capable of plastic deformation. Hooke’s law describes reversible elastic deformation in metals, in which the stress is linearly proportional to the strain. Forces larger than the elastic limit, or heat, may cause an irreversible deformation of the object.
- In general, metals are denser than nonmetals. This is due to the tightly packed crystal lattice of the metallic structure. The larger the amounts of delocalized electrons, the stronger the metallic bonds are.
Terms
- Metal: Any of a number of chemical elements in the periodic table that form a metallic bond with other metal atoms. It is generally shiny, malleable, and a conductor of heat and electricity.
- metallic bond A chemical bond in which mobile electrons are shared over many nuclei; this leads to electrical conduction.
Metallic Properties
In a metal, atoms readily lose electrons to form positive ions (cations). These ions are surrounded by delocalized electrons, which are responsible for conductivity. The solid produced is held together by electrostatic interactions between the ions and the electron cloud. These interactions are called metallic bonds. Metallic bonding accounts for many physical properties of metals, such as strength, malleability, ductility, thermal and electrical conductivity, opacity, and luster.

Understood as the sharing of “free” electrons among a lattice of positively charged ions (cations), metallic bonding is sometimes compared to the bonding of molten salts; however, this simplistic view holds true for very few metals. In a quantum-mechanical view, the conducting electrons spread their density equally over all atoms that function as neutral (non-charged) entities.
Atoms in metals are arranged like closely-packed spheres, and two packing patterns are particularly common: body-centered cubic, wherein each metal is surrounded by eight equivalent metals, and face-centered cubic, in which the metals are surrounded by six neighboring atoms. Several metals adopt both structures, depending on the temperature.
Metals in general have high electrical conductivity, high thermal conductivity, and high density. They typically are deformable (malleable) under stress, without cleaving. Some metals (the alkali and alkaline earth metals) have low density, low hardness, and low melting points. In terms of optical properties, metals are opaque, shiny, and lustrous.
Melting Point and Strength
The strength of a metal derives from the electrostatic attraction between the lattice of positive ions and the “sea” of valence electrons in which they are immersed. The larger the nuclear charge (atomic number) of the atomic nucleus, and the smaller the atom’s size, the greater this attraction. In general, the transition metals with their valence-level d electrons are stronger and have higher melting points:
- Fe, 1539°C
- Re, 3180 °C
- Os, 2727 °C
- W, 3380°C.
The majority of metals have higher densities than the majority of nonmetals. Nonetheless, there is wide variation in the densities of metals. Lithium (Li) is the least dense solid element, and osmium (Os) is the densest. The metals of groups IA and IIA are referred to as the light metals because they are exceptions to this generalization. The high density of most metals is due to the tightly packed crystal lattice of the metallic structure.
Electrical Conductivity: Why Are Metals Good Conductors?
In order for a substance to conduct electricity, it must contain charged particles (charge carriers) that are sufficiently mobile to move in response to an applied electric field. In the case of ionic compounds in water solutions, the ions themselves serve this function. The same thing holds true of ionic compounds when melted. Ionic solids contain the same charge carriers, but because they are fixed in place, these solids are insulators.
In metals, the charge carriers are the electrons, and because they move freely through the lattice, metals are highly conductive. The very low mass and inertia of the electrons allows them to conduct high-frequency alternating currents, something that electrolytic solutions cannot do.
Electrical conductivity, as well as the electrons’ contribution to the heat capacity and heat conductivity of metals, can be calculated from the free electron model, which does not take the detailed structure of the ion lattice into account.
Mechanical properties
Mechanical properties of metals include malleability and ductility, meaning the capacity for plastic deformation. Reversible elastic deformation in metals can be described by Hooke’s Law for restoring forces, in which the stress is linearly proportional to the strain. Applied heat, or forces larger than the elastic limit, may cause an irreversible deformation of the object, known as plastic deformation or plasticity.
Metallic solids are known and valued for these qualities, which derive from the non-directional nature of the attractions between the atomic nuclei and the sea of electrons. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids brittle and subject to fracture when struck with a hammer, for example. A metal, by contrast, is more likely to be simply deformed or dented.
Although metals are black due to their ability to absorb all wavelengths equally, gold (Au) has a distinctive color. According to the theory of special relativity, increased mass of inner-shell electrons that have very high momentum causes orbitals to contract. Because outer electrons are less affected, blue-light absorption is increased, resulting in enhanced reflection of yellow and red light
ASSIGNMENT (10 mks)
1. What is metallic Bond?
2. State three physical properties of metals?
Mr. Kaiwedo Stephen
Submit your assignment to the teacher`s
Email. This email address is being protected from spambots. You need JavaScript enabled to view it.
For further inquiry call him on: 08038960651

